Not all obesity is the same

Obesity is a multifactorial disease with a variety of phenotypes, clinical presentations, and treatment responses.1,2 It is important to distinguish general obesity from genetic obesity caused by rare MC4R pathway diseases. Here you will find the resources you need to help identify some of these rare patients and have confidence in your diagnoses.
Obesity results from factors that disrupt the balance of energy intake (food consumption) and energy expenditure (metabolic rate, thermogenesis, and physical activity). While many environmental factors can influence this balance, our genes also significantly define our body weight. Research has shown that some naturally arising genetic variants are associated with obesity.3-5
General obesity1
- Can be due to:
- Environmental factors
- Multiple common genes interacting together (polygenic)
- Can occur at any age, including later in life
Genetic obesity caused by rare MC4R pathway diseases1,6
- Can be due to rare genetic variants/impairment of gene expression or function
- May be marked by hyperphagia and early-onset, severe obesity, both of which may develop as early as a few months old, but often appear in early childhood
Monogenic obesity (such as POMC, PCSK1, LEPR deficiencies)1
- Presents as rare, early-onset, and severe and is associated with endocrine disorders
- Is mainly due to variants in the MC4R pathway involved in food intake regulation
Syndromic obesity (such as BBS)1
- Is severe obesity associated with additional phenotypes (i.e., dysmorphic features, organ-specific developmental abnormalities)
Hyperphagia
Hyperphagia is a key symptom of several genetic diseases associated with obesity. It is characterized by pathological, insatiable hunger that is differentiated from other overeating behaviours and disorders by its severity and persistence:7
- Longer time to satiety
- Shorter duration of satiety
- Prolonged feeling of hunger
- Severe preoccupation with food and distress if denied food, often associated with abnormal food-seeking behaviours
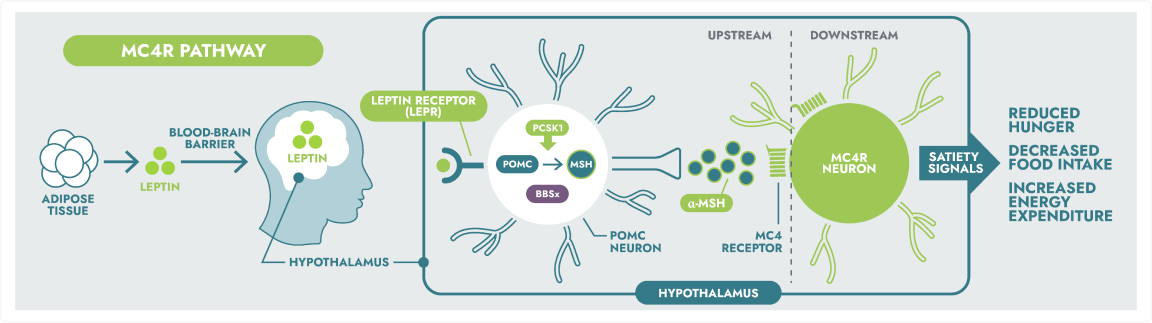
The MC4R pathway
The hypothalamus is a key part of the brain that helps regulate hunger. One way it does this is via the hormone leptin. When leptin falls in response to fasting, the brain triggers actions to restore energy balance. The melanocortin-4 receptor (MC4R) pathway is a key part of this process and a critical regulator of hunger and energy balance which helps maintain stable body weight.3
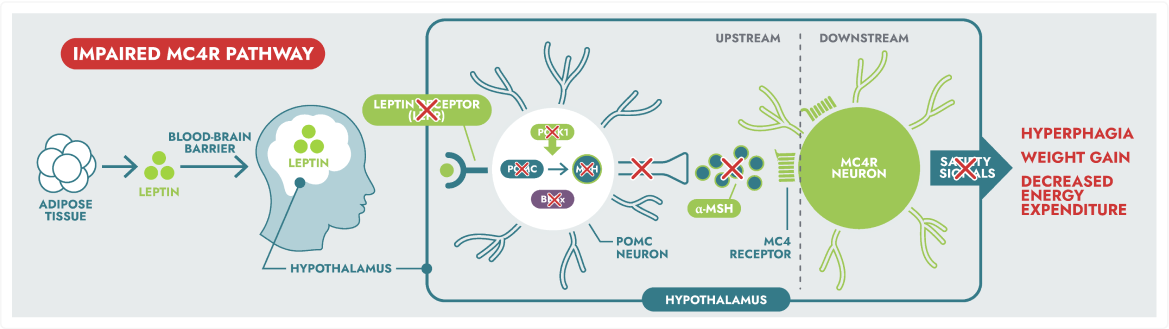
Rare MC4R pathway diseases, which can lead to hyperphagia (pathological, insatiable hunger) and early-onset obesity, are distinct from general obesity.3
MC4R pathway disease states include:
- Rare and severe, early-onset obesity associated with endocrine disorders
- Mainly due to mutations in genes of the leptin-melanocortin pathway, involved in food intake regulation
- Severe obesity associated with additional phenotypes (i.e., dysmorphic features, organ-specific developmental abnormalities)
The hypothalamic melanocortin-4 receptor (MC4R) pathway is a key signalling pathway that regulates hunger, satiety, and energy expenditure.6
Rare genetic variants within the MC4R pathway may result in impaired neuronal signalling, leading to MC4R pathway diseases.1,5
References:
1. Huvenne H et al. Obes Facts. 2016;9(3):158-173. 2. Loos RJF et al. Nat Rev Genet. 2022;23(2):120-133. 3. Fonseca ACP et al. J Diabetes Complications. 2017;31:1549-1561. 4. Littleton SH et al. Mol Diagn Ther. 2020;24:653-663. 5. Yazdi FT et al. PeerJ. 2015;3:e856. 6. Eneli I et al. Appl Clin Genet. 2019;12:87-93. 7. Hampl SE et al. Pediatrics. 2023;151(2):e202206064. 8. Data on file. Rhythm Pharmaceuticals Inc. 9. Vaisse C et al. Cold Spring Harb Perspect Biol. 2017;9(7):a028217. 10. Forsythe E et al. Front Pediatr. 2018. doi:10.3389/fped.2018.00023. 11. Manara E et al. Ital J Pediatr. 2019;45(1):72. 12. Bereket A et al. Obes Rev. 2012;13(9):780-798. doi:10.1111/j.1467-789X.2012.01004.x. 13. Kim JH, Choi JH. Ann Pediatr Endocrinol Metab. 2013;18:161-7.
