IMCIVREE was studied in the
first-ever phase 3 clinical trial dedicated to weight reduction in patients with BBS

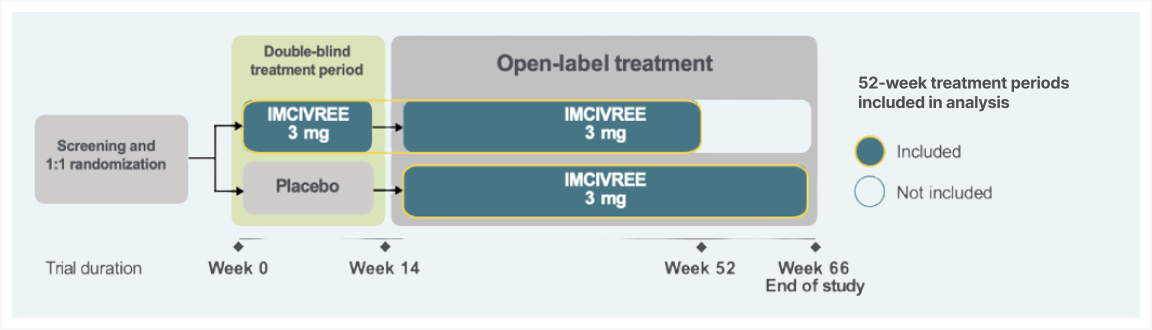
STUDY DESIGN
The efficacy and safety of IMCIVREE for the reduction of weight and hunger in patients aged 6 and older with BBS were studied in a phase 3 trial with a randomized, double-blind, placebo-controlled period.1-3
50% of enrolled patients were children or adolescents who would normally be expected to gain weight as they grow.1,4
The study enrolled patients ≥6 years of age with obesity and a clinical diagnosis of BBS. Adult patients had a BMI of ≥30 kg/m2 and pediatric patients had weight in the ≥97th percentile using growth chart assessments. To maintain the blind during period 1 (14-week placebo-controlled period), dose titration to a fixed dose of 3 mg given subcutaneously once daily was performed during the first 2 weeks of both period 1 and period 2 (52-week open-label period). Efficacy analyses were conducted in 44 patients at the end of period 1 (week 14, placebo-controlled data) and in 31 patients during the active-treatment period, defined as the period from randomization to week 52 in patients initially randomized to IMCIVREE, and from week 14 to week 66 in patients initially randomized to placebo. Analyses of the active-treatment period include patients who had either completed 52 weeks from the start of IMCIVREE treatment or discontinued the study early at the time of the prespecified data cutoff.1
Efficacy in the BBS trial


IMCIVREE was also associated with BMI reduction in pediatric and adult patients with BBS (exploratory endpoint):
- Patients aged from 6 to 18 years achieved a 9.5% mean percent change in BMI after 1 year5
- Patients aged >18 years achieved a ~9.1% mean percent change in BMI after 1 year5
* 95% CI: 18.6, 55.9
† Patients ≥12 years of age who were able to self-report their hunger (n=14) recorded their daily maximal hunger in a diary, which was then assessed by the Daily Hunger Questionnaire Item 2. Hunger was scored on an 11-point scale from 0 (“not hungry at all”) to 10 (“hungriest possible”).5
Safety in the BBS trial
IMCIVREE has a well-established safety and tolerability profile.
Adverse reactions occurring in ≥5% of IMCIVREE-treated patients in the BBS clinical trial.1
| IMCIVREE (n=43)* | |
|---|---|
| Hyperpigmentation disorders† | 63% |
| Injection site reactions‡ | 51% |
| Nausea | 26% |
| Spontaneous penile erection§ | 25% |
| Vomiting | 19% |
| Diarrhea | 14% |
| Melanocytic naevus | 14% |
| Headache | 7% |
| Skin striae | 7% |
| Aggression | 5% |
| Fatigue | 5% |
* 43 patients were treated with at least 1 dose of IMCIVREE; 1 patient initially randomized to placebo withdrew from the study prior to
receiving IMCIVREE and is not included.
† Includes skin hyperpigmentation, hair colour changes, melanoderma, melanocytic naevus.
‡ Includes injection site erythema, pruritus, induration, pain, bruising, edema, reaction, hemorrhage, irritation, mass.
§ n=20 male patients.
Click here to read the Warnings and Precautions associated with IMCIVREE.
Once-daily, subcutaneous injection that can be administered at home1
Titrate IMCIVREE to the recommended dose.
In patients aged 6 to 17 years:1
- The starting dose of setmelanotide is 0.5 mg (0.05 mL) injected subcutaneously once daily for 2 weeks.
- Monitor patients for GI adverse reactions to adjust dosage.
- The dose may be increased by 0.5 mg daily every 2 weeks if tolerated to a maximum dose of 2.0 mg daily.
- If the starting dose is not tolerated, IMCIVREE should be discontinued.
In patients aged ≥18 years:1
- The starting dose of setmelanotide is 1 mg (0.1 mL) injected subcutaneously (SC) once daily (QD) for 2 weeks.
- Monitor patients for gastrointestinal (GI) adverse reactions to adjust dosage.
- The dose may be increased by 0.5 mg daily every 2 weeks if tolerated to a maximum dose of 3.0 mg daily.
- If the starting dose is not tolerated, IMCIVREE should be discontinued.
IMCIVREE should be administered once daily, at the beginning of the day, without regard to meals – there is no food requirement for administration.1
No dose adjustments are needed for patients with mild to moderate renal impairment.1
IMCIVREE is not recommended for use in pediatric patients <12 years of age with severe renal impairment or for use in patients with end stage renal disease.1
For adults and pediatric patients 12 years of age and older with severe renal impairment (eGFR 15 to 29 mL/min/1.73 m2):1
- The starting dose of setmelanotide is 0.5 mg (0.05 mL) injected subcutaneously QD for 2 weeks.
- Monitor patients for GI adverse reactions.
- The dose may be increased by 0.5 mg daily every 2 weeks if tolerated to a maximum of 1.5 mg daily.
- If the starting dose is not tolerated, IMCIVREE should be discontinued.
Dosing considerations:1
- IMCIVREE should be prescribed and supervised by a physician with expertise in obesity with underlying genetic aetiology.
- IMCIVREE should be administered once daily, at the beginning of the day, without regard to meals.
- Select patients for treatment with IMCIVREE who have genetically determined deficiency of POMC, PCSK1, or LEPR or clinical diagnosis of BBS.
- Assess response to IMCIVREE therapy regularly.
- In patients with BBS, evaluate weight loss after 22 weeks of treatment. If a patient has not lost at least 5% of baseline body weight or 5% of baseline BMI for patients with continued growth potential, discontinue IMCIVREE as it is unlikely that the patient will achieve and sustain clinically meaningful weight loss with continued treatment. If a patient has not lost at least 5% of baseline body weight or 5% of baseline BMI for patients with continued growth potential, discontinue IMCIVREE as it is unlikely that the patient will achieve and sustain clinically meaningful weight loss with continued treatment.
- In pediatric patients, evaluate the impact of weight loss on growth and maturation.
Getting your patient started on IMCIVREE
- The Rhythm InTune Enrolment Form is available here or from Rhythm Territory Managers.
- Follow the instructions to complete the form.
- Submit all pages of the completed form via fax to 1-833-350-3887 or email [email protected].
Financial support may be available to eligible patients for whom IMCIVREE treatment is prescribed. For questions on IMCIVREE or how to start a patient, call Rhythm InTune at 1-833-654-2155 Monday-Friday, 8 am to 8 pm ET.
Patient support and resources
In this section, you can find information on educational resources produced by Rhythm. We will continue to update this page with additional resources about rare melanocortin-4 receptor (MC4R) pathway diseases.
for Patients
Enrolment Form
Flashcard
Brochure
References:
1. IMCIVREE (setmelanotide solution for subcutaneous injection) Product Monograph. Rhythm Pharmaceuticals Inc. May 4, 2023. 2. Data on file. Rhythm Pharmaceuticals, Inc. Boston, MA. 3. Haws RM et al. Contemp Clin Trials Commun. 2021;22:100780. 4. Centers for Disease Control and Prevention. 2000 CDC Growth Charts for the United States: Methods and Development. https://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf. Accessed June 14, 2022. 5. Haqq AM et al. Lancet Diabetes Endocrinol. [article and Supplementary Appendix] 2022;10(12):859-868.







