IMCIVREE is the first and only treatment to target an impaired MC4R pathway, a root cause of genetic obesity in people with BBS, POMC, PCSK1, and LEPR deficiency aged 6 and older1,2

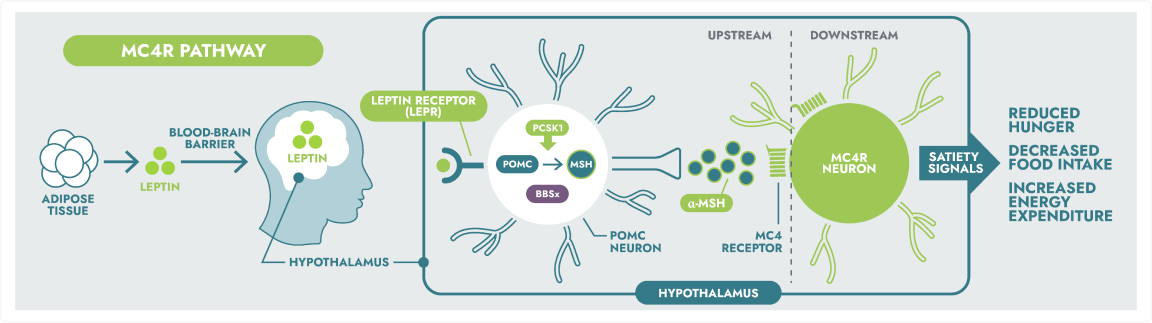
IMCIVREE, an MC4R agonist, is designed to re-establish MC4R pathway activity.1
The hypothalamic melanocortin-4 receptor (MC4R) pathway is a key signalling pathway that regulates hunger, satiety, and energy expenditure.2
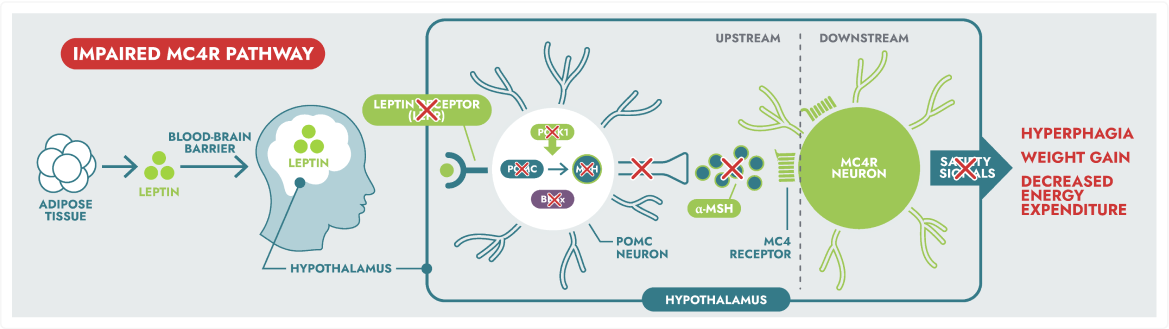
Rare genetic variants within the MC4R pathway may result in impaired neuronal signalling, leading to MC4R pathway diseases.3,4
IMCIVREE is the first medicine to be studied in multiple phase 3 clinical trials dedicated to weight reduction in patients with BBS or POMC, PCSK1, or LEPR deficiency.5,6
IMCIVREE delivered significant and clinically meaningful weight loss over 1 year.5,6
Indications and Limitations of Use1
IMCIVREE (setmelanotide solution for subcutaneous injection) is indicated for weight management in adult and pediatric patients 6 years of age and older with obesity due to:
- Bardet-Biedl syndrome (BBS)
- Genetically confirmed biallelic pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency due to variants interpreted as pathogenic, likely pathogenic, or of uncertain significance
Limitations of Use:
Setmelanotide is not indicated for the treatment of patients with the following conditions as setmelanotide would not be expected to be effective:
- Obesity due to suspected POMC, PCSK1, or LEPR deficiency with POMC, PCSK1, or LEPR variants classified as benign or likely benign
- Other types of obesity not related to POMC, PCSK1 or LEPR deficiency, or BBS including obesity associated with other genetic syndromes and general (polygenic) obesity
Pediatrics and Geriatrics
Pediatrics (<6 years of age):
No data are available to Health Canada; therefore, Health Canada has not authorized an indication for pediatric patients below 6 years of age.
Pediatrics (6 to 17 years of age):
Based on the data submitted and reviewed by Health Canada, the safety and efficacy of IMCIVREE in pediatric patients (6 to 17 years of age) have been established. Therefore, Health Canada has authorized an indication for pediatric use.
Geriatrics:
Clinical studies of IMCIVREE did not include patients aged 65 and over. It is not known whether geriatric patients would respond differently than younger adult patients.
Contraindications:
Patients who are hypersensitive to this drug or to any ingredient in the formulation, including any non-medicinal ingredient, or component of the container.
IMCIVREE is generally well tolerated. Across all patients, the most reported treatment-emergent adverse events were injection site reactions, skin hyperpigmentation, spontaneous penile erection, nausea, headache, diarrhea, abdominal pain, vomiting, melanocytic nevus, back pain, fatigue, depression, asthenia, dizziness, and dry mouth.
Click here to read the Warnings and Precautions associated with IMCIVREE.
References:
1. IMCIVREE (setmelanotide solution for subcutaneous injection) Product Monograph. Rhythm Pharmaceuticals Inc. May 4, 2023. 2. Eneli I et al. Appl Clin Genet. 2019;12:87-93. 3. Yazdi FT et al. PeerJ. 2015;3:e856. 4. Huvenne H et al. Obes Facts. 2016;9(3):158-173. 5. Clément K et al. Lancet Diabetes Endocrinol. 2020;8(12):960-970. 6. Haqq AM, et al. Lancet Diabetes Endocrinol. 2022;10(12):859-868.
